IMS Process
1
Radioactive Water (including tritiated water) + Particles
2
Dissolve, or remove by filtration, Any Particles
3
Radioactive Water (including tritiated water) + Particles
4
Circulate Water
+ Control Chemical Parameter
5
Remove Radioactive Isotopes + Additives so that water can be recycled
CONSTANTS
Temperature = Room Temperature
Atmospheric Pressure = 1atm
Influence on radioactive half-life
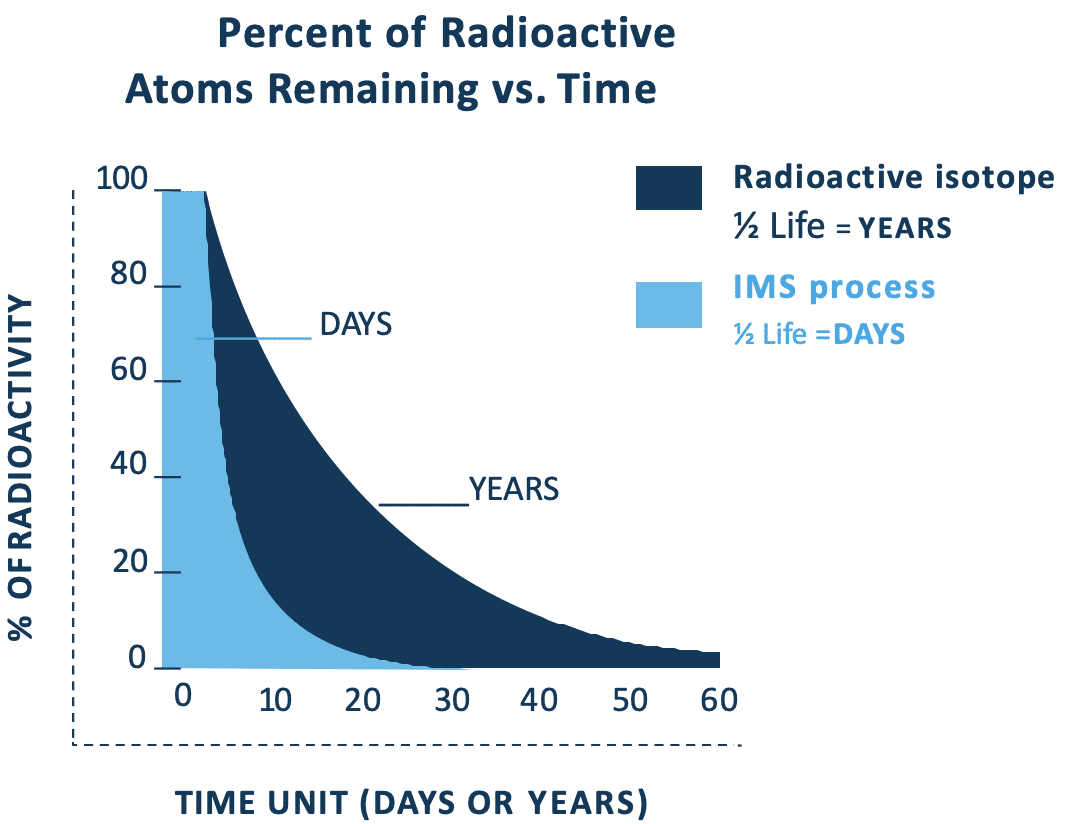
IMS Can Process Isotopes
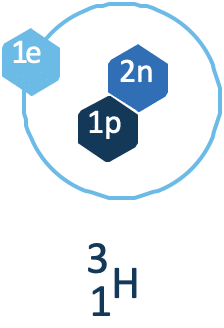
Tritium
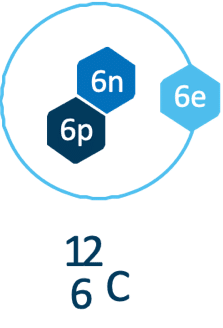
Carbon
Cesium
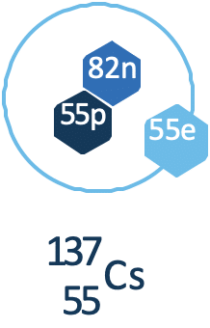
Cobalt
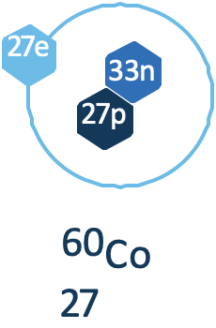
All isotopes can be processed with an adaptation of the process.
BENEFITS OF THE IMS PROCESS
- The IMS process significantly reduces radioactivity emitted by radionuclides, solved in water
- Small amounts of secondary waste and therefore environmentally friendly/Cost effective
- Reduced costs
- Less impact on environment due to avoidance of long term rad waste storage
- Helps to improve acceptance of nuclear energy in modern society
- Future R & D will increase scope of application and allow to solve and process more radionuclides in water.
